|
|||
|
The Big Polymers: RNA
In the big scheme of things we wonder how we got here, the Big Hand. But what was the sequence, what order, what strategy? Was the key to life DNA? Actually - far more likely - RNA. It all has to do with catalysis and control. Replication alone won't get you to where we are. Catalysis The money of chemistry is energy - or heat. We spend heat or energy in chemical reactions. If somebody had given the first guy 30$ to do that job, he gets to keep 20$. If he asked "I will get to keep 30$ if you will wash that window for me for free." then the window would still be dirty. Energy has to be spent for things to proceed. Metabolism, for the most part, is fire. O2 oxidizes carbon material giving off CO2 and H2O just as would a burning log. It probably took some energy input to vaporize (kindle) enough wood so that it could combust. The heat of that combustion kindles in turn and the log burns. To drive the machinery we call life, oxidation proceeds in steps, each stable in itself. Each step can go but needs a boost to get going - to be kindled. For certain chemical reactions two molecules have to be brought together in a very peculiar way for any chance of reaction to take place. Or, it has to occur in a certain environment. One most common case is that oily chemicals need to be in oil to react. The repulse in water. For an oil based reaction to happen in water, we must create a haven, an oily haven, within the water - somehow. Somehow. That IS what a catalyst is. It is the somehow agent. Imagine a jelly donut in a bucket of water. The donut filling is replaced with petroleum jelly (yeah, yuk, but imagine it any way). The outside is water friendly (all sugary) and the inside is oil heaven. Oily reactions could thrive inside the donut, which spits out the final products. This is actually how things work.
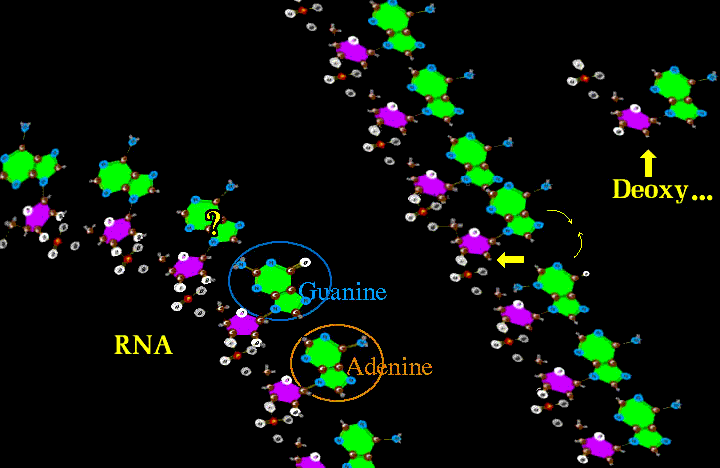
Both RNA (ribose nucleic acid) and DNA (deoxyribose nucleic acid) can replicate. DNA is more gracefully streamlined for replication. But RNA can and does contort and knot up to form twisted blobs with nooks and crannies whose properties can catalyze OTHER chemical reactions, not just replicate.
The Big Hand saw this and allowed the RNA to do the grunt work of assisting in the making of other interesting things and letting RNA reproduce by proxy - by making a copy of RNA as DNA. The DNA just duplicates itself and spins off new RNA while the RNA gets the real work done. But that DNA can get in the way, being so kissy huggy to RNA. Put it somewhere off to the side (nucleus is cool). Now RNA can catalyze, but there are limits as it has such a limited number of properties. To get real catalysis, maybe another proxy? Well if you twist RNA into a hair pin shape and at the bend have three points which can code well over 20 different combinations, then the RNA could do two different jobs. One is to have one for each of the 20 amino acids and a master code RNA which reads sequences of three and recognizes the over twenty amino acid carrier RNAs which drag the unique amino acids into the master sequence.
Why? Because amino acids have so many differing side arms as to be able to create just about any milieu for any chemistry. You just have to get them in the right sequence and let them do their job. All the amino acids have the same key spine : N-C-CO2H They love to link up as N-C-C-N-C-C-N-C-C-N-C-C-N-C-C-N-C-C-N-C-C-... The central carbon has a side piece (one of a selection of about twenty). These side pieces are very varied in chemical properties. Some are acid, some are alkaline, some are oily, some water seeking, some have features for directly binding other things (including other amino acids), some are structured to bend the amino acid, and some are configured to impart stiffness.
Whoa! Big spender! Make something big happen!
But do you have change for a dollar?
So, for example, we have Hexokinase a very mouth-like construction whose teeth grip the six carbon sugar molecule and the ATP and bite one phosphate from the ATP and attach it to the glucose sugar. The muscle of that chomp is driven by ATP (T=Tri=3) the three phosphate power cell which loses only one phosphate. It doesn't drain the ATP battery dead as it leaves ADP (D=Di=2) or the diphosphate form.
Look. See the little ----------^ phosphate? (getting snipped off to put on the glucose) That snip of phosphate springs the ATP=>ADP and that crunches down the big protein mouth to stick certain ring unfriendly atoms right into the glucose that was so comfortable in there when hexokinase was in the open position. The ATTRACTION of the mouth for the glucose is enhanced by the presence of the ATP in there, causing attractors to be active. The ATP does not spring the hexokinase closed UNTIL the glucose sits in place. It is a molecule bear trap. The ATP is both bait and power spring. Neat, huh? Who thought of that??? Uh. Oh. Duh. Now the big obvious question : Why put RNA-DNA in the same section as proteins? Because they are polymers? Starch is a polymer, so is cartilage substrate, and... This section is NOT about polymers but deals with certain polymers. We are talking about CONTROL. If you want to simulate metabolism, at its conclusion, and do so quickly - set a fire! But life isn't about chemical reactions just doing what chemicals do in some broth. Life is getting chemistry to do what it probably wouldn't do - then again - then again, and again ... Life chemistry is the linkage of multiple extreme improbables into a stream of likelihood. Deviation from the shepherded improbables toward primitive naturality is called disease. Pretty philosophic, huh? But true. You see, you've been told that life is just this accidental double helix DNA polymer coming undone - unzipped -and getting dusted with new sub units to create two new polymer copies of the first. Poof. Yeah. Poof, just like fire. No, that's not life, that's runaway chemistry. Look more closely at any zipper and you see that thingy with the pull tab. You unzip something by pulling on that little end gizmo which does the zipper separation. A zipper that unzips itself is - well - embarrassing. Life does not want DNA to just chemically unzip and spew. DNA is heavily chaperoned. It is wrapped on and in and around other substances, proteins, which restrain DNA chemistry. DNA is kept under lock and key. When it does react, that too is chaperoned and restrained by cell chemistry . It is unzipped, not as if by an adolescent in heat, but with decorum, by a protein pull tab, a bit at a time and only so far. There are many steps of many substances in the CONTROLLED and PERMITTED unmasking of DNA. Like a Sabbath reading of the Torah, there is a process and ritual that gives it dignity - cell dignity is health. Ah, but with more steps and procedures come more ways to go wrong (without these steps, though, the whole thing would be consistent rampage of wrongness). The nucleic acids have certain sequences which say "start reading code here." and others which say "stop reading code here". Some of the code is instruction as to how the following code should be read. A sequence might attract an on off switch which sits on the DNA code thus controlling the reading according to concentrations of product that are detected. The cell isn't soup. It is a highly structured factory with assembly lines. There may be proteins which wrap completely around other proteins which wrap around yet another inner protein. Some chemical passed to the center gets passed back through each layer in turn, never in contact with all the other chemistries of that cell. From there? Further improbabilities await - absolutely. |
| [P.O. Home] [Topics] [Muscle] [Basic Science] [Protein-DNA] |
|
|
|
|

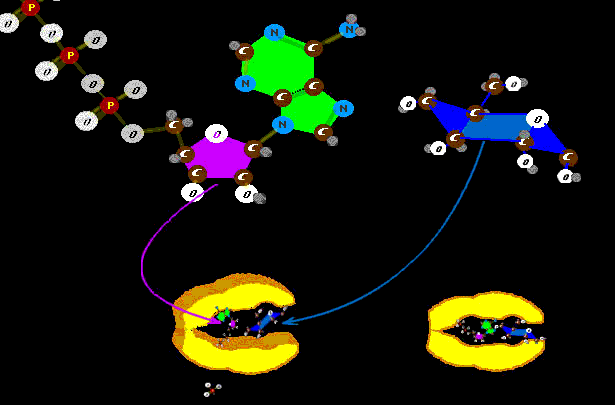
 Once the long sequence is assembled and set free, the side arms of the amino acids cause the coiling and configuration to occur. Proteins then form out
and out machines which have within them several parts which move and reshape according to what else is going on. And they often want money. Chemistry money is energy - energy to make things
that PROBABLY wouldn't happen - happen.
Once the long sequence is assembled and set free, the side arms of the amino acids cause the coiling and configuration to occur. Proteins then form out
and out machines which have within them several parts which move and reshape according to what else is going on. And they often want money. Chemistry money is energy - energy to make things
that PROBABLY wouldn't happen - happen.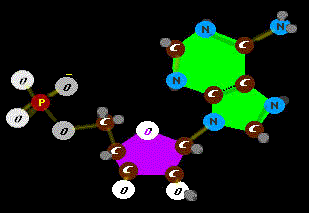 Well, we guess, early on, RNA was carrying the ball. So it is no surprise that the big spender is a basic unit of RNA, a single RNA molecule holding a bribe - an energy
storing phosphate. That highly charged piece repulses and bends the RNA like a leaf spring. So, it stores energy as would a cross bow. Boing! Like a spring in a watch, add
this little beauty to amino acid machines and watch them go - and do things that probably otherwise wouldn't get done. The energy released is a small measured packet,
just sufficient to the task at hand and no more (RNA is not a heavy tipper). But some jobs call for more work and more energy money. Then what?
Well, we guess, early on, RNA was carrying the ball. So it is no surprise that the big spender is a basic unit of RNA, a single RNA molecule holding a bribe - an energy
storing phosphate. That highly charged piece repulses and bends the RNA like a leaf spring. So, it stores energy as would a cross bow. Boing! Like a spring in a watch, add
this little beauty to amino acid machines and watch them go - and do things that probably otherwise wouldn't get done. The energy released is a small measured packet,
just sufficient to the task at hand and no more (RNA is not a heavy tipper). But some jobs call for more work and more energy money. Then what?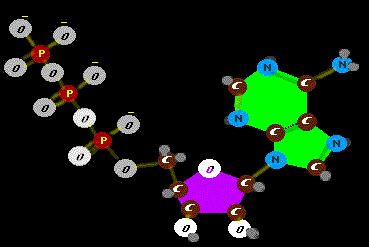
 Ah, yes. Actually this version is a most efficient and compact power cell in protein machines. The phosphate
joins the ribose sugar from two angles with a nifty contortion that allows very fine movement when it releases. It is cyclic
Ah, yes. Actually this version is a most efficient and compact power cell in protein machines. The phosphate
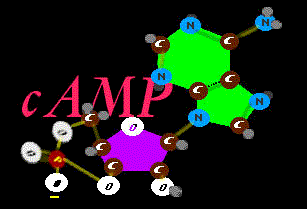
joins the ribose sugar from two angles with a nifty contortion that allows very fine movement when it releases. It is cyclic  cAMP. It is amazing how many
very different chemistries have this unit at the very heart of the action. Interestingly, even the spent batteries, when they are not getting recharged
(reset or retensioned with new phosphate) get utilized as just filler pieces inside more complex protein super constructs. There are a few versions of this using a slightly altered form of the
cAMP. It is amazing how many
very different chemistries have this unit at the very heart of the action. Interestingly, even the spent batteries, when they are not getting recharged
(reset or retensioned with new phosphate) get utilized as just filler pieces inside more complex protein super constructs. There are a few versions of this using a slightly altered form of the